Understanding Deliquescence
This article by Abdel Hadrami originally appared on agadvance.com

Deliquescence by definition means to melt away or to disappear as if by melting. In chemistry, deliquescence refers to the conversion of a solid substance into a liquid as a result of absorption of moisture or water vapour from the air (Fig. 1). Solids always contain impurities, which lower their melting point. Likewise, absorption of water causes a decrease in the normal melting point of a solid. If enough water is absorbed to lower the melting point below room temperature, the solids deliquesce and turn into a liquid.
Nutrients, when they are applied to the soil, onto the seed, or onto the crops, as foliar are taken up according to defined mechanisms. While the body of knowledge regarding nutrient uptake from the soil is quite extensive with defined laws such as diffusion, mass flow, and interception, there still is a lot to be learned about foliar uptake. Foliars are in-crop sprays of liquid solutions that: (i) fulfill nutritional needs during a narrow window of time (7-10 days); (ii) correct micronutrient (Zn, Cu, Mn, B) and a few macronutrient deficiencies (N, P, K, Mg) quickly in a timely manner; and (iii) relieve the crop from an incurred biotic or abiotic stress such as herbicide application, water logging, cold soil or hail damage). Significant changes in terms of crop physiology, growth and development can be observed in response to foliar applied fertilizers.
Foliar uptake involves two major pathways into the leaf, stomata and the polar pores that occur in the waxy cuticle. The key factors influencing the nutrient movement into the leaf are: the charge of the nutrient (cation (+) or anion (-)), particle size, formulation (chlorides, nitrates, or sulphates), and most importantly, the relative humidity at the canopy level. Whether movement is through stomata or the cuticle, foliar fertilizer efficiency depends on the nutrient; formulation, concentration, rate, the wettability of the leaves, and droplets evaporation.
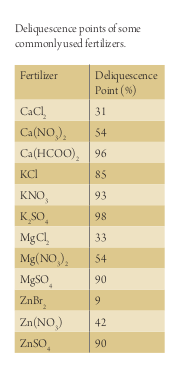
Fertilizer absorbs water from the humidity in the atmosphere at the surface of the leaf depending on the level of hygroscopicity (Fig. 1). For instance calcium chloride (CaCl2) has a very high hygroscopicity of 32% and seeks moisture aggressively while that of potassium sulphate (KSO3) is very low, requiring almost 98% air saturation to dissolve.
The relative humidity (RH) threshold where a fertilizer dissolves into a liquid and below which it remains undissolved (solid) is called the Deliquescence Point. Deliquescence point is a function of RH and temperature, which dictate if foliar uptake by the leaves will occur. When the RH is higher than the deliquescence point, the fertilizer is dissolved, ‘mobile’ and readily taken up by the leaves. On the other hand, when the RH is lower than the Deliquescence Point the fertilizer crystallizes and becomes an ‘immobile’ solid, hence not available to the leaves for uptake. RH fluctuations lead to cycles of deliquescence and crystallization, which contribute to particle agglomeration and caking (Fig. 2).
The efficacy of foliar applied fertilizer depends on deliquescence properties, the laws of thermodynamics, and the transport in the plants. Many processes are involved including the application technique (sprayer nozzle characteristics, deposition of the droplets, wind speed during and after the application.), the retention by the leaf surface (wetting characteristics of the leaves, the contact angles of the droplets with the leaves, the spreading of the sprayed product, the use of surfactants), the mobility and concentration on the leaf surface (degree of evaporation, point of deliquescence, relative humidity), the permeability of the leaf surface (cuticular and stomata penetration), and the mobility in the plants through the phloem.
When a fertilizer solution is applied onto the leaves, the nutrient concentration in each droplet is rather low (Fig. 3). This concentration becomes higher as the droplet evaporates reaching a maximum just before the liquid turns back into a solid (Fig. 3). The early morning or evening dew increases the moisture level at the canopy re-dissolving the fertilizer and making it more readily available for foliar absorption. For example, foliar sodium nitrate has a deliquescence point of 73% so absorption of the product will cease as soon as the RH drops below 73% and be remobilized when the RH rises above 73%. The concentration of the nutrient in the droplet will decrease as the RH increases.
To guarantee a nutrient remains in a prolonged dissolved state to maximize plant uptake; the use of deliquescent products, which are substances with a strong affinity for moisture, is important. These include, but are not limited to, chloride forms of calcium, magnesium, and zinc, potassium carbonate, potassium phosphate, ferric ammonium citrate, potassium hydroxide and sodium hydroxide. The rule of thumb is that chlorides have a lower deliquescence point than those of nitrates and sulphates (Table 1).

Timing foliar applications to the dew point is important to maximize their absorption by the leaves. However, leaf burn is inversely proportional to the deliquescence of the products. The faster uptake of the nutrients with lower deliquescence can result in more leaf burn. While chlorides are superior in getting into the leaf, they burn more than nitrates, and nitrates more than sulphates.
Foliar applied fertilizers are fast acting and can be combined with other treatments such as herbicides and fungicides provided a compatibility test is conducted prior to use. On the other hand, the actual amount of nutrient delivered by foliar applied fertilizers is very low, multiple applications may be required for major deficiencies, especially in case where the nutrient is immobile within the plant (i.e., boron).
Always remember, the form of the applied nutrient is critical to its ability to move into the leaf. Weather and plant health are factors, but the deliquescence point of the nutrient being applied is the main determining factor affecting its movement into the leaf, and the amount of leaf burn experienced.
