Spray Water Quality: Hard Water vs Soft Water
Have you ever wondered if you have hard or soft water? Perhaps you’ve heard or been a part of a conversation regarding water being hard or soft and wondered what exactly that means. You may have also noticed scaling and deposits left by hard water on the interior or exterior of plumbing and pipes. Maybe this didn’t bother you because, after all, water is water, isn’t it? But, consider this — what if the water quality that affects your drinking and shower water also makes a difference in the chemistry of what you are spraying on your crops?
The Basics
Water in its natural form doesn’t contain any minerals and is considered “soft.” However, when it contains a high concentration of dissolved minerals, especially calcium (carbonate) and magnesium, it becomes “hard.” Water often picks up these minerals when passing through materials such as limestone. Calcium and magnesium also increase the pH of the water, with magnesium having twice as much impact as calcium.
Water from wells and dug-outs, often used in crop spraying and in many mixes on the farm, is hard water. Even water from cities and municipalities is not as soft as you may think.
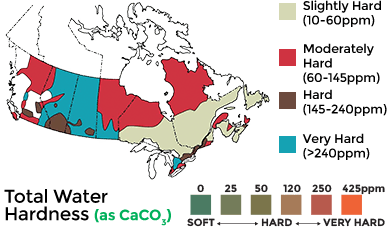
According to the Water Quality Association, water hardness is listed in grains per gallon (gpg) or in parts of million (ppm) and interpreted as follows:
Soft: 0 – 1 grains per gallon (gpg); 0 parts per million (ppm)
Slightly Hard: 1.1 – 3.5 gpg; 10 – 60 ppm
Moderately Hard: 3.6 – 7 gpg; 60 – 145 ppm
Hard: 7.1 – 10.5 gpg; 145 – 240 ppm
Very Hard: Over 10.5 gpg; Over 240 ppm
The map below shows various areas and their water hardness based on the above interpretation guidelines. The table below shows each location and the corresponding recorded average for water hardness. These figures don’t replace an actual water hardness test but should serve as a reference.
What effect does water have on crop spraying?
More than ever before, water quality in Canada is becoming a topic of discussion when it comes to applying foliar fertilizers or pesticides. Whether it is well water, retained water from rain and snow melt or other natural sources, it seems that Canada’s calcareous soils influence its mineral contents and hardness.
Hard water often has a high pH and its continuous application tends to increase soil pH. Many herbicides, fungicides and insecticides require an acidic solution for better efficacy. Some of their active ingredients are negatively charged molecules, prone to “lock-up” and fixation by calcium and magnesium. These end up forming insoluble complexes that sink to the bottom of the tank, reducing efficacy of weed, disease and insect control. High pH also affects the permeability of the leaves to the sprayed nutrients or chemistries.
What can you do?
Water conditioners such as pHix® and softeners such as SopHtener95® are available to condition water, rendering it soft before the addition of nutrients and chemistries and thereby maximizing uptake and efficacy. Choose pHix® or SopHtener95®, depending on application. pHix® is recommended for conditioning water for pre-burn and desiccation, while SopHtener95® is ideal for in-crop spray to soften the spray water and increase the solution chelation properties and the spread of the droplets onto the leaves.
OMEX has been leading the industry by providing farmers with water conditioners and softeners. Since its introduction, pHix® has been the industry standard for reducing water hardness, lowering the pH and improving the efficiency of burn-off and desiccation with a variety of pesticide tank partners. SopHtener95® has recently been added to our line-up of products, for use with in-crop sprays. Speak to your OMEX representative about how to best manage your spray water hardness.
