Free Up Your Phosphorus
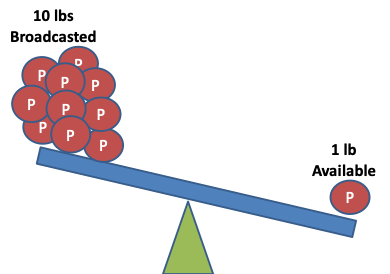
Broadcasting fertilizer is a practice commonly used to maximize soil fertility, with advantages for managing the seeding operation early in the spring. However, this practice is detrimental to the efficiency of phosphorus, as it exposes it to either run-off in wet springs or, early tie-up or both. In this post we will examine the factors that can impair or improve phosphorus availability for uptake by plants.

Phosphorus’s availability for plant uptake can be affected by several factors. The first and most important factor is the reaction of dissolved phosphorus with soil constituents within the soil solution to form less soluble compounds. This process is known as sorption, where phosphorus adheres to certain components of soil particles, hence becoming unavailable to plants for uptake.
Desorption refers to the opposite process that allows phosphorus to leave soil particles and go back into solution. This mechanism is moisture-, pH- and temperature-dependent and often requires the help of microorganisms from the rhizosphere.
In the topsoil, phosphorus is also prone to losses due to water or wind. Soluble phosphorus can then be lost in surface run-off or through deep leaching under some circumstances if precipitation exceeds soil water holding capacity.
Forms of phosphorus in the soil
Phosphorus is usually concentrated near the soil surface, thanks to the cycling of phosphorus through the vegetation and its deposition on the soil surface in undisturbed systems, or to the application of fertilizer in cultivated soils.
Phosphorus in the soil is present in three different pools that differ in their availability to crops.
- First, the readily available pool of phosphorus that is in soil solution;
- second, the pool of phosphorus that is exchangeable and can rapidly be released from the soil to replenish the soil solution, and finally,
- the relatively slowly available phosphorus that requires weathering and microbial activity over a period of time.
The soil solution portion of phosphate is the lowest and the most limiting for growth and development.
Soil pH greatly influences phosphorus availability (read our previous blog on soil pH). At high pH levels (alkaline soils), calcium and magnesium phosphate develops, while at low pH levels (acidic soils) aluminum and iron phosphate forms. These low-solubility products remove phosphorus from the soil solution, rendering phosphorus less available to the growing crops.
Factors affecting phosphorus availability
As we know, the balance between mineralization and immobilization of phosphorus in soil is strongly influenced by temperature, pH and moisture.
- Temperature. Mineralization of organic phosphorus typically starts at temperatures >12°C up to a maximum at above 30°C. This range coincides with the optimum growth and development temperatures of many phosphorus-solubilizing bacteria such as those present in Agriflora Soil, with an arsenal of phosphatases able to turn organic phosphorus from an unavailable to available form.
- pH. Phosphorus is prone to tie-up whether at acidic or alkaline pH, however, the process of mineralization relying on the activity of microorganisms in the rhizosphere can vary depending on the microorganisms thriving either in low or high pH. The phosphatases from these microorganisms helps release more phosphorus from the organic pool, complementing the effect of organic acids released by the crop in the root zone to break bounds and free up inorganic phosphorus to meet crop demand.
- Moisture. It affects root growth, uptake of phosphorus and mineralization. After a rain, microbial growth increases, which immobilizes phosphorus among other nutrients into the microbial biomass but also leads to an increase in organic phosphorus leaking from viable microbial cells. Once the soil dries out, the microbial population decreases, leading to a significant release of inorganic and organic phosphorus from dead microbial cells and plant tissues. This cycle of desiccation and re-wetting of the soil facilitates the process mineralization and the continuation of availability of phosphorus for the crop.
Maximizing phosphorus availability
OMEX has developed a series of technologies and products that enhance phosphorus availability and reduce its tie-up. For instance, the use of TPA has proven to be effective at reducing the tie-up of phosphorus to calcium in high pH soil, making both phosphorus and calcium available to the crop. Using the product in low pH soil protects phosphorus against tie-up with either aluminum or iron. Similarly, using carboxylates helps replicate the process of plants releasing organic acids into the root zone to solubilize phosphorus and make it more available.
The combination of these technologies in a Starter P in-furrow applied liquid elevates its efficacy and widespread use regardless of soil conditions. Also, the use of these starters with humate and biological products such as Agriflora Soil help increase phosphorus mineralization and availability.
How can we at OMEX help?
OMEX offers a wide range of Primers, Starters, Foliars and PGRs, Biologicals and Biostimulants that contain micronutrients, molecules, co-factors, activators and technologies able to help stabilize, reduce losses and prevent the tie-up of phosphorus. OMEX Primers contain sufficient amounts of PK and micronutrients to fulfill the need of the crop until the root system starts tapping into the banded fertilizers. Starters are formulated with TPA and Carboxylate to maximize availability and reduce to phosphorus tie-up and leaching and Foliars are formulated with highly available nutrients for maximum uptake and translocation to prevent and correct deficiencies or reduce the effect of biotic and abiotic stress. Whatever your goals and growing conditions may be, OMEX is here to help you grow healthy and high-quality crops that yield to their full potential. Talk to your local Ag Retailer or get in touch with your OMEX representative to learn more about OMEX products and technologies and their fit on your farm.
