
TPA


Nutrient Uptake Efficiency Enhancer
ANALYSIS (5-15-5)
What is It?
- TPA stands for Thermo Poly Aspartate
- Highly polymerized and negatively charged beta protein.
- Biodegradable and lasts 120 days in the soil.
- The product is available in 10L jugs, 450L and 1000L IBC’s. Also available with PGRs.
When & Why Use It?
- Used in starter fertilizer to reduce the binding of phosphorus with Ca2+, Fe3+ or Al3+.
- Applied in alkaline soils with high content of Ca2+ and in acidic soil with high levels of Fe3+ or Al3+.
- By reducing the tie-up, TPA increases nutrient availability.
What to Expect?
- Improved root development and growth.
- Better nutrient availability and uptake visible in tissue tests.
- Increased tuber count in potatoes with an even size.
- Improved marketable yield in potatoes.
Size Options

Tote

10L
Application Guidelines
- Apply 1-2 L/ac of TPA with in-furrow applied liquid fertilizer.
- TPA can be injected into the irrigation water (micro jet or drip).
- TPA may be used to reduce corrosion in liquid fertilizer tanks.
- It can be applied in combination with a foliar fertilizer program.
Findings
2016

Manitoba 2016 | Clay loam: lockport
2015
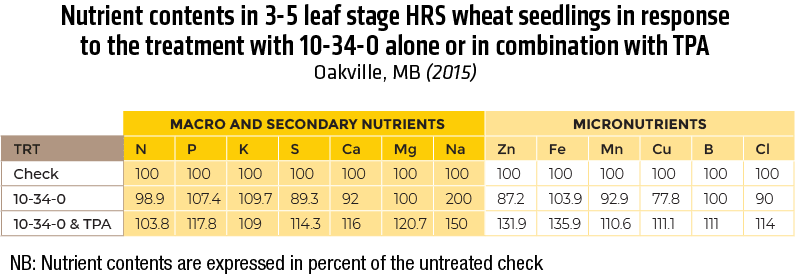
2015 – In Clay-loam soil (Lockport)



2015 – In Heavy clay soil (Oakville)



2015 – In Heavy clay soil (Oakville)



2015 – Sandy soil: McGregor; Heavy Clay: St François-Xavier
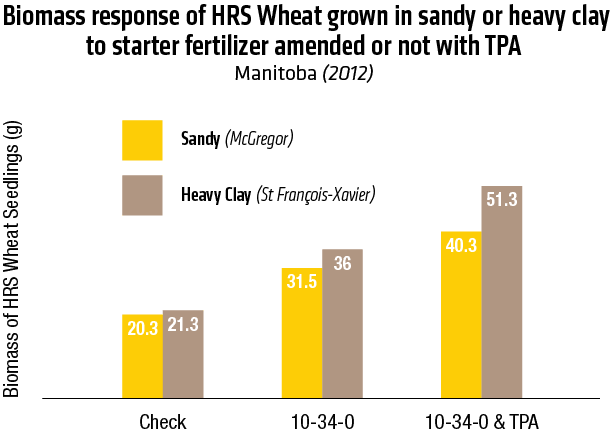
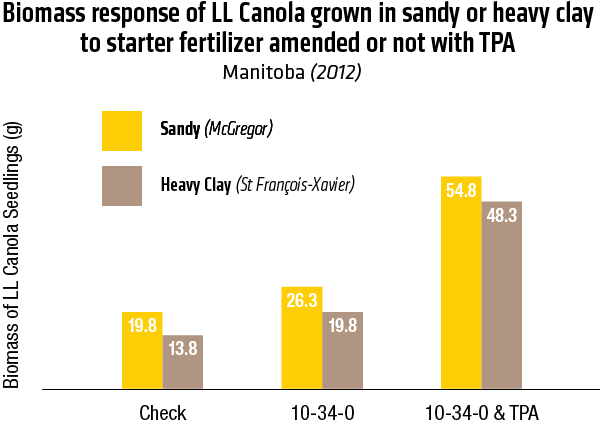
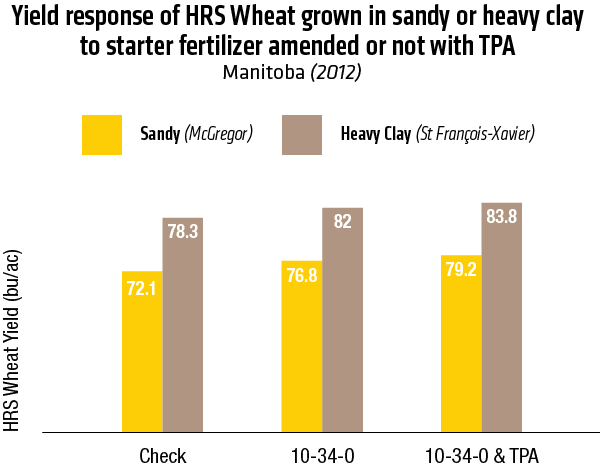
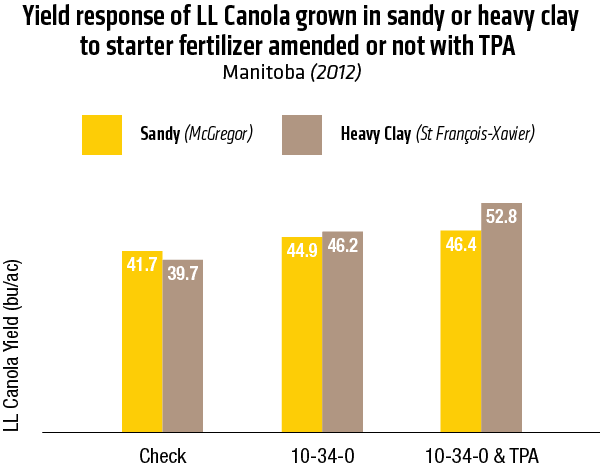
2015 – In Heavy clay soil (St François-Xavier)



2015 – In sandy soil (McGregor)



2015 – McGregor, Manitoba 2012

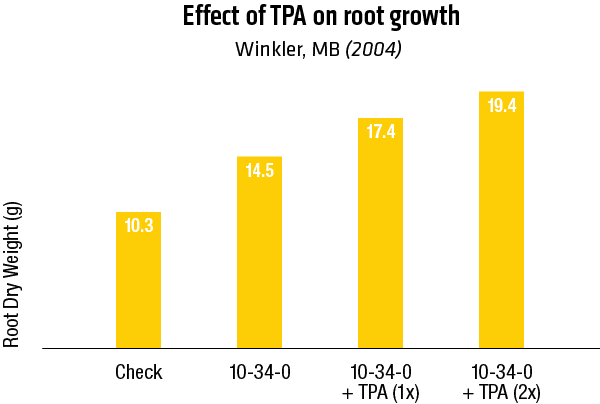
2004 – Winkler, Manitoba


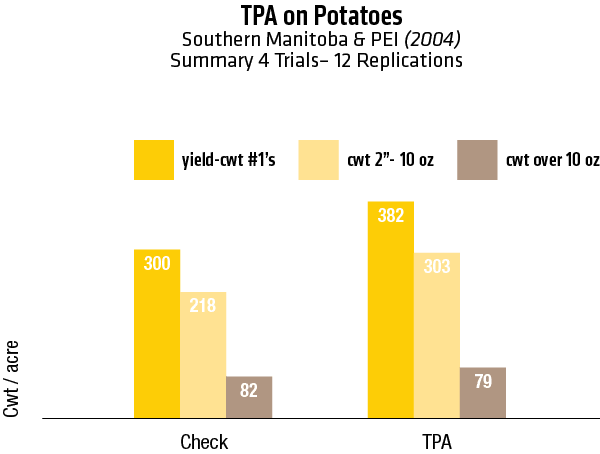
2003
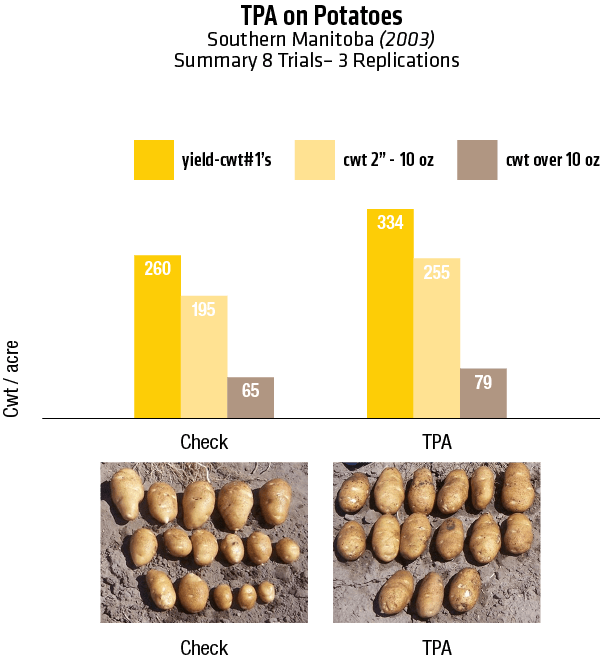
2003 – Winkler, Manitoba


Additional


8 Stolons & Hooks – TPA & Starter


Impregnation of TPA on dry blend



