Preventing Nitrogen and Phosphorus Leaching
Nitrogen and phosphorus losses can be a big concern for the farmer, both environmentally and economically. Most losses are unintentional, but that doesn’t mean they’re unavoidable. In this post, we will explore how OMEX can help you prevent nitrogen and phosphorus leaching through a nutrient management plan designed to stabilize, reduce losses of N and prevent tie-up of P.
Before we dive into the topic of preventing nutrients from leaching out of the soil, let’s distinguish “availability” from “solubility.” Nutrients in the soil should be plant-available, not water-soluble. The latter exposes nutrients to leaching – especially in light-textured or heavily weathered soils.
Nitrogen
Nitrogen is a component of organic matter and is required by plants in relatively large amounts. It is an essential constituent of amino acids, proteins, DNA, RNA and chlorophyll, and affects many processes including photosynthesis, growth and development along with reproduction and seed set.
For instance, dark green colored leaves mean high levels of chlorophyll due to a sufficient nitrogen supply, versus leaves that are a lighter shade of green would indicate an inadequate nitrogen supply.
Nitrogen Cycling in the Soil
Looking at the nitrogen cycle (Figure 1), seven forms are involved: atmospheric N gas (N2), ammonium (NH4+), ammonia (NH3), nitrate (NO3-), nitrite (NO2-), nitrogen oxide gases (NO, N2O) and organic N. Losses of nitrogen can occur due to erosion, denitrification, volatilization and/or leaching.
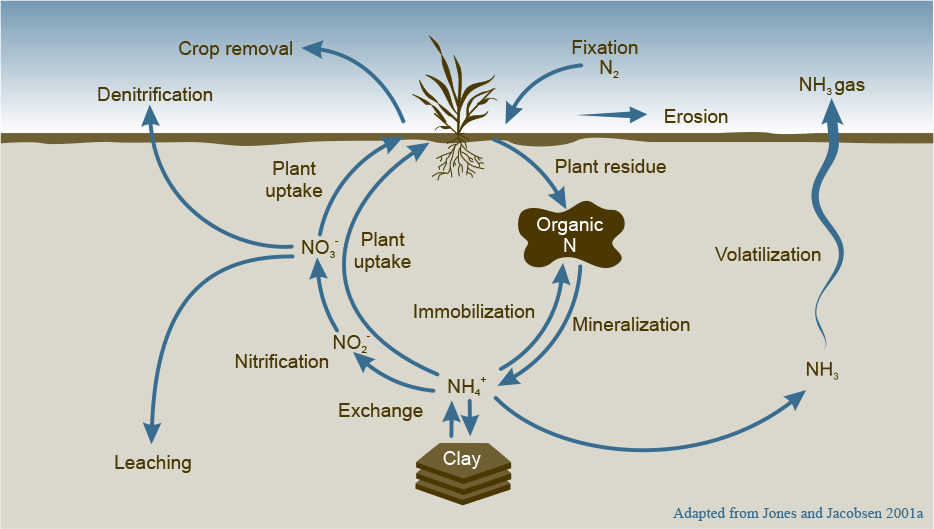
Solubilizing Nitrogen and Preventing Leaching
To solubilize nitrogen in the soil (make it more soluble), nitrate or ammonium nitrogen need to be converted into amino acids through the process of bacterial mineralization. This requires carbon and some key nutrients such as sulfur and molybdenum. An ideal mineralization with a maximum solubility of nitrogen often occurs when the ratio of carbon to nitrogen to sulfur is 300:10:1.
So, when using UAN and ATS, use 10 times more UAN than ATS to provide sulfur to quickly convert nitrogen into microbial amino acids. The most important component for the process is carbon, which can be supplied in form of humic acid.
As long as nitrogen is kept out of the nitrate form that is leachable, most of the other forms are either plant-available or commonly protected using nitrogen stabilizers such as urease or nitrification inhibitors. For instance, staging the application of nitrogen rather than applying the full recommended rate at seeding helps reduce losses.
Improving mineralization by using biologicals (e.g. Agriflora Soil), or encouraging biological activity through the use of humic acid, TPA or carboxylates, helps to increase the organic nitrogen pool and minimize leaching. The deficiency of molybdenum or other conditions that reduce the translocation of sugars in the plant (Mg or B deficiency) can also be a source of poor conversion of nitrate in the soil, exposing it to leaching.
Phosphorus
Phosphorus is the element of life, as it is the source of storage and transfer of energy in forms of adenosine tri-/di-phosphate (ATP/ADP). This high-energy molecule drives virtually every biochemical reaction and process in the plant. Phosphorus is also an important structural component of plants as it is part of the structure of nucleic acids, proteins, enzymes and phospholipids.
Phosphorus Cycling in the Soil
Phosphorus is the second most limiting nutrient worldwide in intensive farming areas, and in Prairie soils, in particular. In many soils, P, and not N, is the most limiting plant nutrient. Phosphorus deficient soils often have an acidic pH with very low organic matter and virtually no organic residues returned to the soil. Less than 20% of the total P content of surface soils (0-6”) is plant-available. Figure 2 below shows three pools that represent the sources of phosphorus in the soil: mineral P, soluble P and organic P.
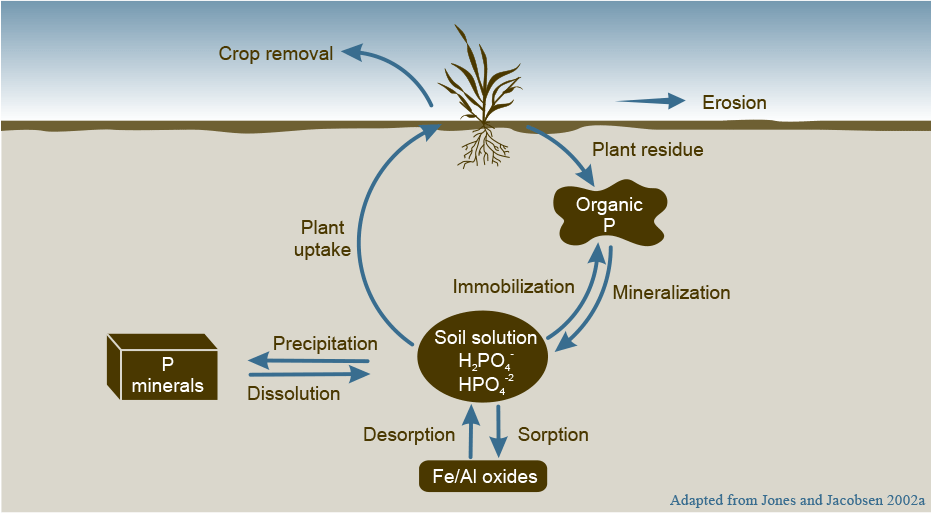
- Mineral P constitutes 20–85% of total soil P, depending on the soil and its organic matter composition.
- Soluble P is a negligible percentage of total phosphorus in the soil, but it is the only P that plants can use. Soluble P can take the form of H2PO4– and HPO42-. These two forms co-exist in proportion to each other depending on soil pH. At a pH of 6.2, the ratio H2PO4-: HPO42– is 10:1, while at a of pH 7.2 the ratio is 1:1 and, at a pH of 8.2, the ratio is 1:10.
- Organic P represents 15–80% of the total P. Soil organic matter typically contains C-N-P-S in a ratio of 140:10:1.3:1.3. Knowing the organic matter content provides an estimate of Organic P, which is typically cleaved by phosphatases produced by either microorganism present in the rhizosphere or secreted by plant roots.
Soluble Phosphate
Two forms of soluble phosphate exist: ortho- and poly-phosphate. Think of it as a metal chain where one link represents orthophosphate and multiple attached links form the polyphophate. Plants absorb phosphorus in the orthophosphate form (e.g. C3, Nutriboost, uPtaKe IC 5-25-5, uPtaKe IC 8-32-5). Only a very small amount of phospohorus is available naturally in the soil at any given time in its orthophosphate form.
Generally, about 90% of phosphorus applied at seeding becomes tied up within 20–40 days from application. Crops generally only absorb 5–10% of what is applied in a given seeding season.
In acidic soils, phosphorus reacts with iron, aluminum and manganese, while in alkaline soils, phosphorus reacts with calcium and magnesium. Optimal availability of phosphorus occurs at soil pH of 6–7.
Precipitated phosphorus cannot be absorbed by the roots and must be released through microbial activity; this activity is often repressed when high levels of phosphorus have been applied. In an optimized system, a staged application of phosphorus is required alongside the use of technologies and molecules that are able to reduce phosphorus tie-up (e.g. TPA) or increase the stimulation of microbial activity (e.g. Biostimulants, Humic, Agriflora Soil).
Phosphorus can leave the soil in two different ways: (i) as a soluble P shortly after application or (ii) with soil particles during a run-off. That’s why broadcasting phosphorus is not, and never was, a good idea.

How can we here at OMEX help?
OMEX offers a wide range of products that can help stabilize, reduce losses of nitrogen and prevent the tie-up of phosphorus. Depending on your soil conditions and fertility plan, OMEX Plant Health Professionals can review the best options for you from OMEX’s lineup of Primers, Starters, Foliars, PGRs, Biologicals and Biostimulants. These formulations contain micronutrients, molecules, co-factors, activators and technologies that can help stabilize and reduce losses of nitrogen and prevent the tie-up of phosphorus.
For example, OMEX Primers contain a sufficient amount of PK and micronutrients to fulfill the need of a crop until its root system can start tapping into banded fertilizers; Starters are formulated with TPA and Carboxylate to maximize availability and reduce to phosphorus tie-up and leaching; and Foliars are formulated with highly available nutrients for maximum uptake and translocation to prevent and correct deficiencies or reduce the effect of biotic and abiotic stress. Our Plant Health Professionals can help you evaluate the best choice for your farm. Whatever your requirements, OMEX is here to help you grow healthy, high-quality crops that yield to their full potential. Talk to your local Ag Retailer or get in touch with your OMEX representative to learn more about OMEX products and technologies and their fit on your farm.
