Dealing with Acidic Soils
Soil pH is a key factor in farmland as it controls availability of nutrients, microbial activity and crop productivity. Before delving into what causes soils to become acid and the steps to take to treat and correct acidic soil, we must first establish what is considered an optimal pH for crop production.
For most prairie crops, a soil pH range of 6.0 to 8.0 is suitable for optimal growth and development. Soils with pH ranging from 5.6 to 6.0 are considered moderately acid, while strongly acid and very strongly acidic soils have pH ranging from 5.1-5.5 to <5.0, respectively. Crops have difficulty establishing and show a decline in productivity and yield in soils with a pH below 6.0.
What Causes Soils to become Acid?
Multiple factors can be at the origin of soil acidity:
- The parent substrate from which the soil developed can be a source of acidity. For instance, soils developed from land with high organic matter containing conifers or with high contents of iron or aluminum tend to be acidic.
- Nitrification of ammonium fertilizers yields hydrogen ions, which increase acidity.
- Acid rain contains nitric and sulfuric acids; both have low pH.
- Added elemental sulfur oxidizes to form sulfuric acid.
- Soils in areas with large amounts of rainfall tend to be acidic because the water leaches basic cations such as calcium, magnesium, potassium and sodium out of the soil profile, leaving room for acidic cations such as hydrogen and aluminum.
- Carbonic acid formed from carbon dioxide and water acidifies soils in high-precipitation areas.
- Acidic soils tend to be high in iron and aluminum oxides, as they are the slowest minerals to weather in soil. Aluminum in these increasingly acidic soils is solubilized and will combine with water to release additional hydrogen ions contributing to further acidity.
- With plant uptake of cations, hydrogen becomes more prevalent in the soil profile due to root exudation of organic acids.
Consequences of Soil Acidity
- Low pH caused by soil acidity increases the solubility of zinc, manganese, iron and aluminum, which can lead to rapid accumulation and toxicity.
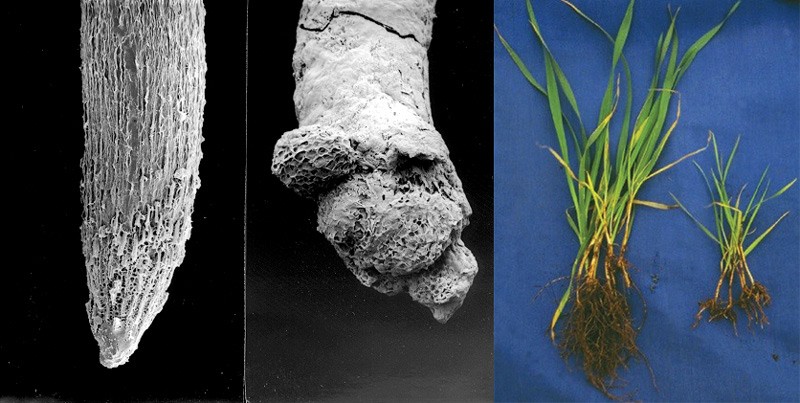
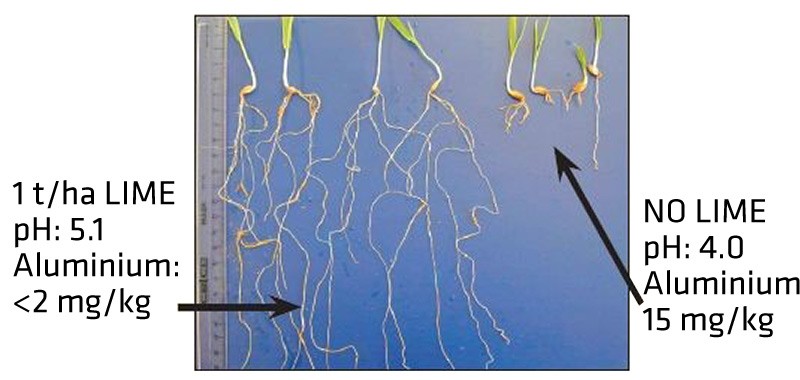
Figure 1. Damage to the root system, growth and development of wheat caused by aluminum toxicity in acidic soil. - The high levels of aluminum and iron in acid soil reduces phosphorus availability by causing tie-up.
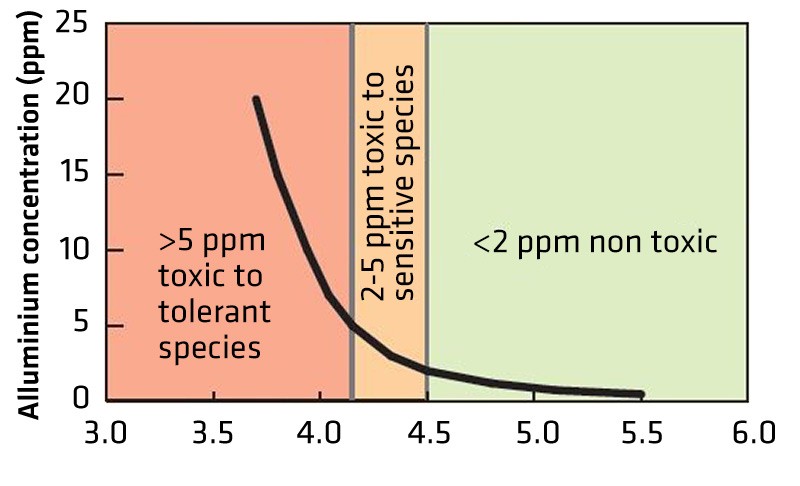
Figure 2. Relationship between soil pH and aluminum toxicity. - Molybdenum becomes less available to plants in acidic soils, which affects nitrogen and phosphorus uptake and utilization, restricts root growth and reduces nodulation in pulse crops.
- Acidity in the soil affects microbial activity, the decomposition of organic matter and mineralization.
- Under acid conditions, calcium, magnesium, and potassium deficiencies become more pronounced.
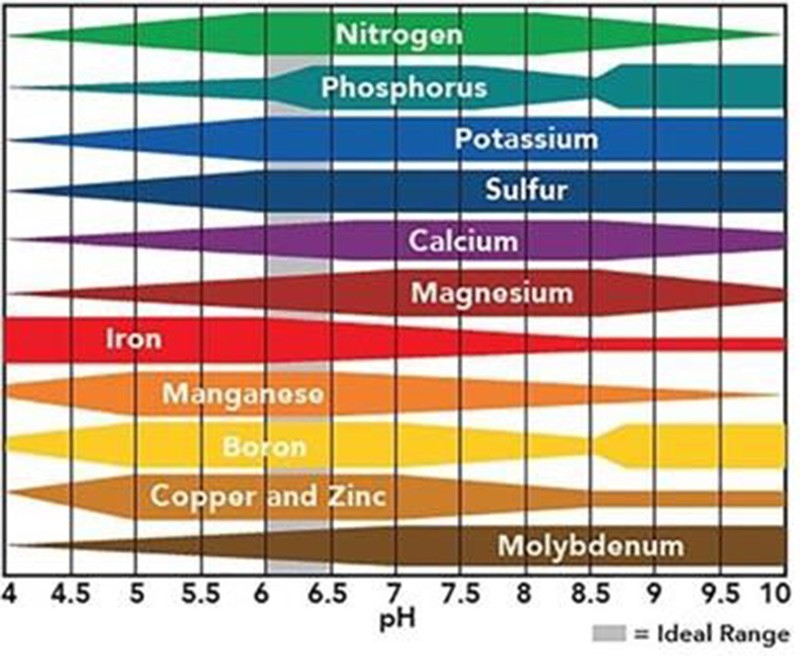
Figure 3. Nutrient availability in the soil based on pH. At low pH, molybdenum, nitrogen, phosphorus, calcium and magnesium become less available, while iron, zinc, manganese and aluminum become more available.
Soil acidity can be improved, and the pH of the soil increased, by the addition of calcium and/or magnesium. This can be in the form of calcic or dolomitic lime, wood ash, CKD or other calcium carbonate-derived amendments. Each of these amendments has both benefits and drawbacks in terms of effectiveness, purity, speed of action and cost.
Lime is most effective at neutralizing acidity when it is incorporated or tilled into the soil to the full depth of the root zone. The amount of lime material required depends on the following factors:
- The pH of the untreated soil and the desired pH to grow the crop;
- the amount of soluble and exchangeable acidity;
- crop tolerance to acidity/alkalinity;
- cost of the lime amendment will affect the choice of product;
- amount of organic matter in the soil;
- and the type of clay present in the soil.
The more carbonate equivalent in the lime amendment, the less product is needed; timing of application depends on the selected source of lime.
OMEX Has the Solution
OMEX has developed several products that complement liming and can help mitigate the effects of aluminum toxicity.
Pulse Primer, Pulse Pak and Primer Soybean – Applied to seeds prior to sowing, these calcium-based seed primers protect the emerging radicle from the toxic effect of aluminum, encourage nodulation, and provide a sufficient amount of molybdenum unavailable in acidic soils.
TPA – This in-furrow liquid Starter improves efficiency of phosphorus, diminished due to aluminum or iron tie-up. TPA is formulated with a patented Thermo Poly Asparte molecule with a higher CEC that breaks the bonds between aluminum/iron and phosphorus, rendering the latter more available to the plants.
In addition, we offer various sources of calcium and magnesium to help increase soil pH, and humates that can be used to improve soil’s organic matter content and biological activity.
Whether or not you have acidic soils, your OMEX representative can guide you in the selection of the right products for your crop’s needs. Your rep can provide you with a nutrient management strategy that incorporates our wide range of seed-applied Primers, in-furrow Starters, in-crop Foliars and other specialty products to help you meet your yield goals, under a wide range of conditions.
